You may be entitled to recover compensation through the filing of a Cartiva Lawsuit, and our law firm can help. We are seeking Cartiva Implant Lawsuits in all 50 states. Please click the button below for a Free Cartiva Implant Failure Consultation or call our Cartiva Implant Lawyers toll-free 24 hrs/day for legal advice by dialing (866) 588-0600.
Table Of Contents
- Cartiva Toe Implant Lawsuit Overview
- Latest Cartiva Implant Lawsuit Updates
- FDA Reports and Statistics
- Cartiva Toe Implant Injuries & Side Effects
- Do You Qualify for a Cartiva Toe Implant Lawsuit?
- Reasons Leading to Cartiva Toe Implant Failure
- Statute of Limitations for Cartiva Toe Implant Lawsuits
- FAQs
- 1. What is the Cartiva Synthetic Cartilage Implant?
- 2. What are the allegations in Cartiva Implant lawsuits?
- 3. What is big toe arthritis?
- 4. What were the treatment options before Cartiva?
- 5. What are the symptoms of big toe arthritis?
- 6. Can I join a class action lawsuit for Cartiva Implant failures?
- 7. How much compensation could I receive in a Cartiva lawsuit?
- Get a Free Cartiva Toe Implant Lawsuit Evaluation With Our Lawyers
Cartiva Toe Implant Lawsuit Overview
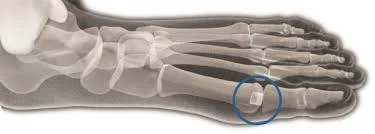
Recent lawsuits allege that the Cartiva toe implant, a synthetic cartilage device used to treat arthritis in the big toe, was defectively designed and is prone to failures. These failures can cause pain, the need for revision surgery, and other serious injuries.
The implant received FDA approval in 2016 based on the MOTION clinical study, but patients have reported complications that were not disclosed by manufacturers.
Latest Cartiva Implant Lawsuit Updates
- July 25, 2024 – Several voluntary dismissals have been entered in early Cartiva implant failure lawsuits, giving plaintiffs the option to refile their claims at a future date [1].
- May 31, 2022 – Gina Neil entered a complaint in the Western District of North Carolina alleging defects in the Cartiva implant [2].
- March 14, 2022 – Gabriel Gesmer filed a lawsuit in the Northern District of California claiming that the Cartiva implant did not meet federal standards before it was brought to market. Gesmer also alleges that Cartiva Inc. knew or should have known about the device’s risks, having received thousands of adverse event reports associated with the product [3].
- 2018 – Wright Medical Group purchased the rights to the Cartiva synthetic cartilage implant for $435 million, expecting success comparable to the implant’s positive clinical trials [4].
Also Read:
FDA Reports and Statistics
The FDA approved the Cartiva Synthetic Cartilage Implant in August 2016, making it the first synthetic cartilage device approved by the FDA.
The approval was based on the MOTION clinical study, a 236-patient, multi-center, prospective, randomized study comparing Cartiva SCI to fusion surgery – the largest study ever conducted for this condition [5].
According to lawsuits, manufacturers knew of more than 144 adverse event reports involving loss of toe mobility, pain, and high failure rates but failed to report the increasing rate of failures and complications to the FDA as legally required.
Cartiva Toe Implant Injuries & Side Effects
Patients who received the Cartiva synthetic cartilage implant have reported numerous serious complications and side effects:
- Motion issues: Difficulty moving the toe joint
- Significant pain: Persistent or worsening pain in the surgical area
- Bone damage: Loss of bone, bone spurs, and degraded cartilage
- Implant failure: Subsidence (implant caving into the bone), fracture, slipping, or shrinking
- Nerve damage: Resulting in additional pain and mobility issues
- Device failure: High failure rate requiring revision surgery
Do You Qualify for a Cartiva Toe Implant Lawsuit?
You may qualify for a Cartiva Implant lawsuit if:
- You had surgery to implant a Cartiva toe implant.
- You experienced failure of the implant.
- You were required to undergo additional surgeries.
- You have not been diagnosed with cancer, rheumatoid arthritis, Crohn’s Disease, or Multiple Sclerosis.
Evidence Required for a Cartiva Toe Implant Lawsuit
- Medical documentation of your Cartiva implant surgery
- Records of complications, failures, or revision surgeries
- Documentation linking your injuries to the Cartiva implant
Damages You Can Recover
- Medical costs: Including corrective procedures, therapies, medications, and other medical needs following an adverse outcome
- Income losses: Compensation for lost wages due to pain or lost mobility in the big toe
- Pain and suffering: Damages for serious pain, lost function, depression, and other non-financial losses
Reasons Leading to Cartiva Toe Implant Failure
There are 4 specific reasons why Cartiva Implants are prone to failure:
- Smooth implant surface: The Cartiva Implant is made of an inert substance with very little reactivity in the body. However, the device is very smooth on all surfaces, which creates a functional flaw in the design.
- Violation of the Subchondral Plate: The subchondral plate is the hard bone underlying the cartilage. Loss of support from the subchondral plate with no alternative means of support places the Cartiva Implant into the soft metaphyseal bone, making it prone to impaction failure.
- Lack of Osteointegration: Osteointegration, or ingrowth of bone into the implant, is an essential aspect of implant stability. This mechanism is lacking with the Cartiva implant.
- Placement in Bone Too Soft for Support: The supporting bone is too weak to provide meaningful, long-term support to resist the load applied to the implant.
Statute of Limitations for Cartiva Toe Implant Lawsuits
There are statutes of limitations that vary by state for filing Cartiva implant lawsuits, so it’s important to act quickly to preserve your legal rights.
See all related defective medical device lawsuits our lawyers have covered.
FAQs
1. What is the Cartiva Synthetic Cartilage Implant?
The Cartiva Synthetic Cartilage Implant (SCI) is intended to treat painful big-toe arthritis, the most common arthritic condition in the foot. It’s a biocompatible, biomedical polymer device that features physical properties similar to those of articular cartilage.
2. What are the allegations in Cartiva Implant lawsuits?
Lawsuits allege that Cartiva toe implants were defectively designed due to problems with the PVA material. They further claim that Wright Medical knew or should have known about the high failure rate and other problems with the Cartiva joint replacement before FDA approval but failed to disclose these risks.
3. What is big toe arthritis?
Also known as stiff big toe or hallux rigidus, arthritis of the big toe joint occurs when the cartilage covering the ends of the bones in the big toe joint (metatarsophalangeal joint) is worn down. This causes the bones in the joint to begin rubbing together, resulting in stiffness and pain.
4. What were the treatment options before Cartiva?
Before Cartiva’s approval, the standard of care involved fusing the bones in the arthritic joint with screws and plates. While fusion proves effective for eliminating pain, it permanently prevents movement of the joint.
5. What are the symptoms of big toe arthritis?
Symptoms include stiffness and pain in the big toe joint, difficulty moving the big toe up and down, inflammation and swelling around the joint, and pain that worsens with increased activity.
6. Can I join a class action lawsuit for Cartiva Implant failures?
Depending on the circumstances, you may be able to join a class action or pursue an individual lawsuit. If a high volume of Cartiva implant cases get filed, they may be consolidated into multidistrict litigation (MDL).
7. How much compensation could I receive in a Cartiva lawsuit?
Compensation varies by case but could include medical costs for corrective procedures, income losses due to disability, and damages for pain and suffering and other non-financial losses.
Get a Free Cartiva Toe Implant Lawsuit Evaluation With Our Lawyers
Time is limited to pursue legal action in Cartiva Implant cases. Statutes of limitations vary by state, making it critical to act promptly.
The Medical Device Litigation Group at our law firm is an experienced team of trial lawyers focusing on plaintiffs’ representation in Cartiva Toe Implant Lawsuits. We are handling individual litigation nationwide and currently accepting new adverse event reports involving Cartiva implants in all 50 states.
Our services include:
- Free, confidential consultations.
- No upfront costs or fees.
- Payment only if we win your case.
References
- https://www.classaction.org/cartiva-toe-implant-failure-lawsuits
- https://www.aboutlawsuits.com/wp-content/uploads/2022-05-31-Neil-Complaint.pdf
- https://dockets.justia.com/docket/california/candce/4:2022cv01609/393002
- https://www.globenewswire.com/news-release/2018/08/27/1556765/0/en/Wright-Medical-Group-N-V-Enters-Into-Definitive-Agreement-to-Acquire-Cartiva-Inc.html
- https://www.opnews.com/2016/08/fda-approves-cartiva-synthetic-cartilage-implant/12867

 Published by
Published by