Please click the button below for a Free Confidential Case Evaluation or call us toll-free 24 hrs/day by dialing (866) 588-0600 to discuss your legal options. Our Paragard attorneys are filing Paragard lawsuits and investigating potential Paragard cases now.
If you or a loved one experienced broken IUD, device embedding, organ perforation, or serious complications following Paragard IUD use, you may be entitled to pursue compensation.
At Schmidt & Clark, LLP, we are dedicated to helping individuals who have suffered due to defective Paragard IUDs. Our experienced legal team is here to guide you through the process and fight for the compensation you deserve.
Contact Schmidt & Clark, LLP today for a free, no-obligation consultation.
Table Of Contents
- Paragard Lawsuit Overview
- Latest Paragard Lawsuit Updates
- FDA Reports and Statistics
- Paragard Injuries & Side Effects
- Do You Qualify for a Paragard Lawsuit?
- Paragard Recall Information
- Statute of Limitations for Paragard Lawsuits
- FAQs
- 1. What is Paragard?
- 2. What’s the problem with Paragard?
- 3. How is Paragard removed?
- 4. What treatment is needed if Paragard breaks?
- 5. How is Paragard different from Mirena?
- 6. Has there been a Paragard class action lawsuit?
- 7. What are Paragard lawsuits alleging?
- 8. What damages could I be awarded in a Paragard lawsuit?
- Get a Free Paragard Lawsuit Lawsuit Evaluation With Our Lawyers
Paragard Lawsuit Overview
Paragard lawsuits allege that the copper IUD device is defective and that manufacturers failed to provide adequate instructions to doctors about proper removal techniques. The primary allegations focus on the device’s arms breaking during removal, leaving fragments in the uterus. The litigation currently includes over 2,283 cases consolidated in multidistrict litigation.
The FDA has received over 1,600 reports of Paragard breaking, with more than 700 classified as “serious.”
Latest Paragard Lawsuit Updates
- February 2025: The court set the first bellwether trial for December 1, 2025, and the second for February 2, 2026, marking a significant milestone in the litigation’s progress. Numerous lawsuits were dismissed due to statutes of limitations, narrowing the MDL to cases within the allowable time frame. The scheduling of trials represented a crucial step toward resolving the core issues of the Paragard litigation [1].
- October 2024: For the first time in five years, the MDL saw a net decrease in pending cases, losing 49 lawsuits while only 12 new cases were filed in early October. This reduction was primarily due to dismissals and procedural rulings as the court narrowed the pool of active cases. The change signaled a shift in the pace and focus of the litigation as it matured [2].
- September 2023: Judge May issued an amended scheduling order, extending deadlines for fact and expert discovery in response to the complexity of the MDL. The first bellwether trial was scheduled for October 28, 2024, giving parties additional time to prepare. This update reflected the ongoing growth and evolving nature of the litigation [3].
- May 2023: By mid-May, more than 180 new claims were filed, bringing the total number of cases to nearly 2,000. Judge May implemented a hybrid approach for bellwether selection, allowing both sides to choose cases for the first trials. These steps marked a significant expansion in the litigation and set the stage for the next phase of coordinated discovery and trial preparation [4].
- February 2023: Judge May provided formal guidance on the bellwether case selection process, outlining a procedure for choosing representative cases for early trials. The court established that 10 cases would be selected as bellwethers, with trials initially targeted for 2024. This process was designed to test legal theories and case value, guiding future settlements and litigation strategy [5].
- January 2023: Judge Leigh Martin May appointed retired Judge M. Gino Brogdon Sr. as mediator for the Paragard MDL. The mediator’s role was to facilitate settlement negotiations between plaintiffs and defendants and help resolve cases without the need for trial. This appointment reflected the court’s intent to manage the complex litigation efficiently and encourage early resolution [6].
- December 16, 2020 — The Paragard lawsuits were consolidated into a federal multidistrict litigation (MDL No. 2974) in the U.S. District Court for the Northern District of Georgia. This consolidation aimed to streamline pretrial proceedings for the growing number of cases alleging that the Paragard copper IUD broke during removal and caused injuries. The MDL centralizes discovery, motions, and settlement discussions under one judge, improving efficiency and consistency for plaintiffs and defendants alike [7].
FDA Reports and Statistics
According to FDA reports, the agency has received over 1,600 reports of the Paragard IUD fracturing, with more than 700 of those reports considered serious. In 2023 alone, the FDA received at least 934 adverse incident reports involving Paragard breaking during removal, which is three times the number reported in 2020.
A 2015 study published in the Open Journal of Clinical & Medical Case Reports examined multiple cases of the Paragard IUD device breaking upon surgical removal, concluding that “the possibility of its breakage should be recognized by clinicians [8].
The FDA first approved Paragard in 1984 [9]. However, despite multiple adverse event reports, the device remains available on the U.S. market, with only one batch recalled in 2019 due to sterility concerns [10].
Paragard Injuries & Side Effects
The Paragard IUD has been linked to numerous severe complications, particularly when the device breaks during removal.
- Breakage during removal: Plastic arms becoming rigid and breaking off, leaving fragments in the uterus
- Migration issues: Device expulsion, embedding in the uterus, or migration to other organs
- Pregnancy complications: Ectopic pregnancy (fertilized egg growing outside the uterus)
- Perforation injuries: Perforation of the uterine wall/cavity or other organs
- Severe complications: Inflammation/allergic reactions, internal bleeding, scarring to organs
- Necessary procedures: Hysterectomy, revision surgery, cervical dilation
- Life-threatening conditions: Copper toxicity, pseudotumor cerebri, or death from related injuries
 Do You Qualify for a Paragard Lawsuit?
Do You Qualify for a Paragard Lawsuit?
You may qualify for a Paragard lawsuit if:
- You were implanted with a Paragard IUD.
- You experienced serious side effects including device breakage during removal, perforation, embedding, or migration.
- Your injuries required medical treatment, surgery, or hospitalization.
- You can provide medical documentation linking your injuries to the Paragard IUD.
Evidence Required for a Paragard Lawsuit
- Medical records confirming your Paragard implant
- Documentation identifying your specific implant
- Medical records detailing your specific injury
- Evidence linking your injury to the Paragard device
Damages You Can Recover
- Current and future medical expenses
- Lost wages and reduced earning capacity
- Pain and suffering
- Loss of consortium
- Other non-economic damages
 Paragard Recall Information
Paragard Recall Information
According to the FDA, despite cases alleging that the Paragard T 380A copper IUD is defective, it remains available for sale on the U.S. market. However, one batch of Paragard was recalled in 2019 due to sterility concerns.
Statute of Limitations for Paragard Lawsuits
The time limit to file a Paragard IUD lawsuit, known as the statute of limitations, varies by state. It’s essential to contact a lawyer promptly to ensure your claim is filed within the legal timeframe to preserve your rights.
FAQs
1. What is Paragard?
Manufactured and marketed by The Cooper Companies, Paragard is a copper IUD used for preventing pregnancy in women. It’s made of a plastic base with a copper coil wrapped around it. The copper creates an inflammatory reaction in the uterus that interferes with sperm.
2. What’s the problem with Paragard?
The Paragard IUD is linked to serious side effects and complications due to the device’s reaction in the body, removal problems, or the IUD breaking. The primary issue is that the plastic arms can become rigid over time and break during removal.
3. How is Paragard removed?
Paragard must be removed in an authorized healthcare provider’s office. The provider will use forceps to grasp the IUD’s strings and gently pull. The IUD arms should fold upward as it is withdrawn from the uterus.
4. What treatment is needed if Paragard breaks?
Women who experience breakage typically require invasive treatments including surgery to remove broken IUD pieces, uterine incision, total hysterectomy, general anesthesia, and ultrasound imaging before surgery.
5. How is Paragard different from Mirena?
Both are IUDs, but Mirena uses levonorgestrel (a synthetic hormone) and is effective for 5 years, while Paragard is hormone-free and works for up to 10 years.
6. Has there been a Paragard class action lawsuit?
To date, only individual lawsuits have been filed, which have been consolidated into multidistrict litigation (MDL). Unlike a class action, MDL allows all cases to remain separate.
7. What are Paragard lawsuits alleging?
Plaintiffs allege that Teva Pharmaceuticals and Cooper Companies created a defective contraception device and failed to warn patients and doctors about the risk of the device fracturing upon removal, despite marketing it as easy to remove.
8. What damages could I be awarded in a Paragard lawsuit?
You may be entitled to compensation for current/future medical bills, loss of wages, reduced earning capacity, loss of consortium, and non-economic damages such as pain and suffering.
Get a Free Paragard Lawsuit Lawsuit Evaluation With Our Lawyers
Time is limited to pursue legal action regarding Paragard injuries. The statute of limitations varies by state, so prompt action is essential to preserve your legal rights.
Our firm offers:
- Free, confidential consultations
- No upfront costs or fees
- Payment only if we win your case
References
- https://www.lawsuit-information-center.com/paragard-iud-lawsuits-payout-value.html
- https://www.motleyrice.com/medical-devices/paragard-lawsuit
- https://www.gand.uscourts.gov/sites/gand/files/LMM2974_Doc249.pdf
- https://dockets.justia.com/docket/multi-district/jpml/MDL%20NO.%202974/1355959
- https://www.gand.uscourts.gov/20md2974
- https://www.courtlistener.com/docket/397351/in-re-paragard-iud-products-liability-litigation/
- https://medtruth.com/articles/legal-developments/paragard-iud-lawsuits-transferred-to-georgia/
- http://jclinmedcasereports.com/articles/OJCMCR-1049.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018680s060lbl.pdf
- https://www.fda.gov/media/129526/download

 Published by
Published by 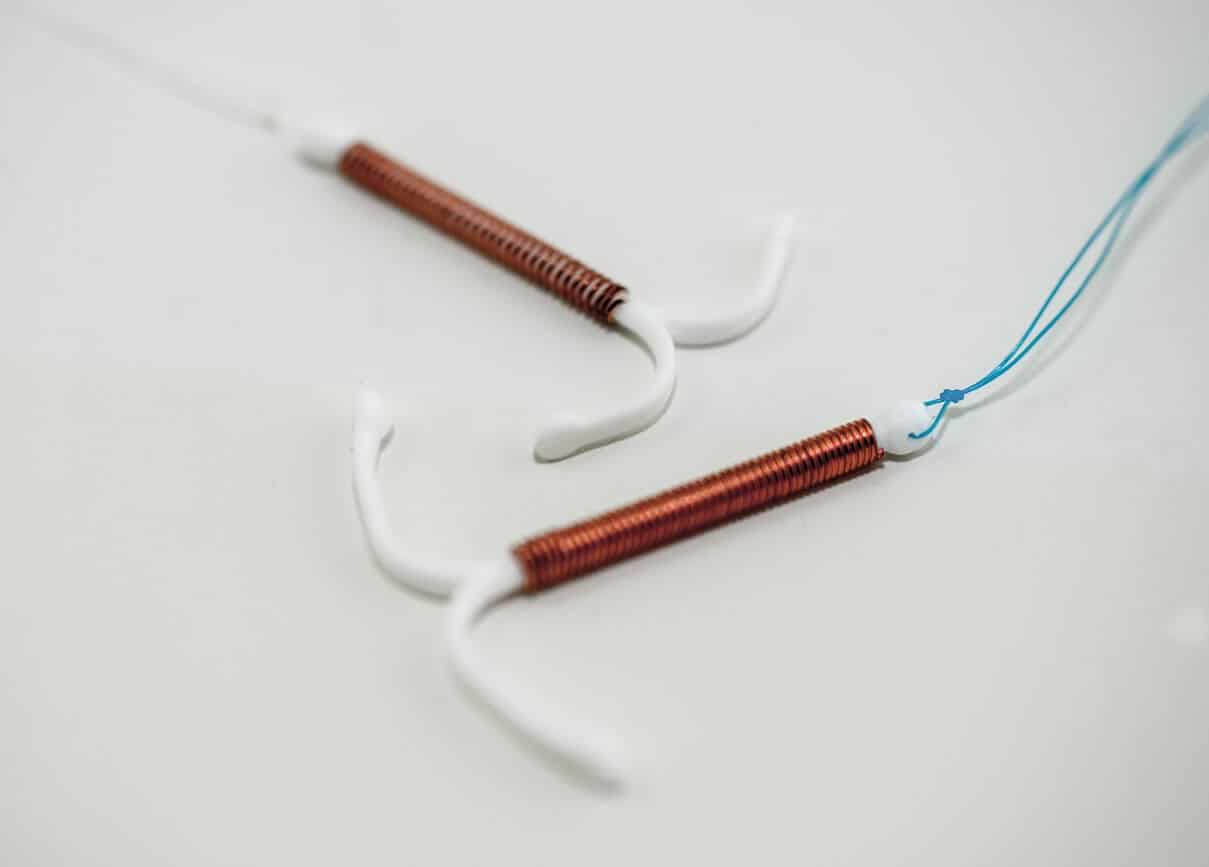
 Do You Qualify for a Paragard Lawsuit?
Do You Qualify for a Paragard Lawsuit? Paragard Recall Information
Paragard Recall Information