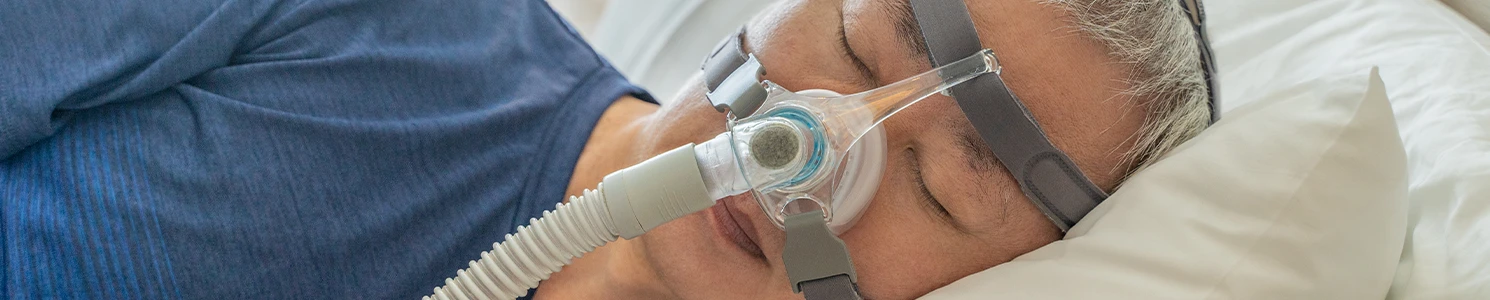
CPAP Cancer Lawsuit Overview
CPAP cancer lawsuits target Philips Respironics for manufacturing sleep apnea machines containing polyester-based polyurethane (PE-PUR) foam that can degrade and release carcinogenic particles and gases into users' airways.
The primary allegations claim that Philips knew about these dangers but failed to warn consumers or recall affected devices in a timely manner.
In June 2021, Philips recalled approximately 2-3 million CPAP, BiPAP, and ventilator devices due to these serious health risks, with an estimated two-thirds of these recalled devices used in the United States.
The FDA has classified this as a Class I recall, their most serious category, indicating "a reasonable probability that the use of these products will cause serious injuries or death."
Latest CPAP Lawsuit 2026 Updates
- April 2025 – Philips has allocated $575 million for settlement of CPAP cancer claims in the multidistrict litigation (MDL) pending in Pennsylvania federal court. This proposed settlement would resolve approximately 8,500 personal injury claims from users who developed cancer after using recalled devices.
- February 2025 – A bellwether trial resulted in a $15 million verdict for a plaintiff who developed lung cancer after using a Philips DreamStation CPAP machine for five years. The jury found that Philips knew about the foam degradation issues but failed to warn patients.
- December 2024 – The FDA announced enhanced testing requirements for all CPAP manufacturers, focusing specifically on the safety of sound abatement materials and ensuring they do not degrade under normal use conditions.
FDA Reports and Statistics on CPAP-Related Health Risks
The FDA and other health agencies have compiled significant data regarding the health risks associated with recalled Philips CPAP devices:
- According to FDA data, there have been over 98,000 Medical Device Reports (MDRs) associated with the PE-PUR foam breakdown in Philips devices, including 346 reports of death.
- Independent testing commissioned by the FDA found that the degraded foam releases harmful chemicals including toluene diamine (TDA), toluene diisocyanate (TDI), and diethylene glycol, all of which have known carcinogenic properties.
- Approximately 22 million Americans have moderate to severe sleep apnea, with an estimated 3.5 million using CPAP therapy [1].
- Studies indicate that users of affected devices had, on average, a 16% higher rate of cancer diagnosis compared to users of non-recalled CPAP machines.
- The FDA has classified the Philips recall as a Class I recall, their most serious type, indicating "a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death" [2].
"These issues can result in serious injury, which can be life-threatening, cause permanent impairment, and require medical intervention to prevent permanent damage." the U.S. Food and Drug Administration said in its Safety Communication
CPAP Injuries & Side Effects
If you've been using a Philips CPAP sleep breathing device, you'll know you've been exposed to foam particles if you experience:
Skin irritation
- Eye irritation
- Breathing problems
- Airway Inflammation
- Respiratory tract irritation
- Chest pressure
- Sinus inflammation
- Headache
- Dizziness
- Nausea
- Vomiting
- Hypersensitivity
Users of recalled Philips CPAP machines may experience a range of health effects, from minor irritation to life-threatening conditions due to the inhalation of toxic foam particles and gases.
Short-Term Health Issues
- Respiratory Issues: Coughing, asthma, airway inflammation, chest pressure, and respiratory tract irritation
- Inflammatory Reactions: Sinus inflammation, ear inflammation, nose inflammation, and throat inflammation
- Hypersensitivity: Skin irritation, eye irritation, and allergic reactions
- Neurological Symptoms: Headache, dizziness, cognitive difficulties
- Gastrointestinal Issues: Nausea and vomiting
Serious Long-Term Health Risks
- Cancer:
Brain, breast, bladder, kidney, lung, liver, lymphatic, nasal, prostate, pancreatic, stomach, rectal, testicular, and thyroid cancers
- Blood Cancers: Leukemia, Non-Hodgkin's Lymphoma, Multiple Myeloma, and Hematopoietic cancer
- Severe Respiratory Conditions: Pulmonary fibrosis, lung damage, Reactive Airway Disease (RAD), and Acute Respiratory Distress Syndrome (ARDS)
- Organ Failure: Respiratory failure, heart failure, and chemical poisoning leading to organ damage
- Other Serious Conditions: Pleural effusion, pneumonia, and severe systemic inflammatory response
Why is PE-PUR Foam in Philips CPAP Machines Dangerous?
PE-PUR foam in Phillips CPAP machines is dangerous because when it disintegrates, it releases carcinogenic gases.
PE-PUR foam that hasn't broken down is harmless, but it can cause mild to life-threatening consequences once it's broken down.
Philips' lab determined that degraded foam releases harmful chemicals such as:
Toluene diamine — A chemical used for dyes and explosives. It can cause genetic defects, cancer, and lowered fertility.
- Toluene diisocyanate — Used for foam and coatings. It can cause eye and skin irritation and can have fatal consequences when inhaled.
- Diethylene glycol — Used to make chemicals that irritate skin and is toxic if inhaled.
- Dimethyl diazene — Used to make other chemicals.
Polyurethane foam is known to be harmful. Fire marshals call this kind of foam solid gasoline. It's especially dangerous in Philips CPAP machines, as they can send carcinogenic air straight into the body.
Do CPAP Machines Cause Cancer?
Most CPAP machines don't cause cancer, but some brands that use certain carcinogenic materials in their products might be contributing to it.
It's estimated that 22 million Americans have moderate to severe sleep apnea. People who have sleep apnea have issues breathing during sleep — their breathing stops and starts repeatedly. CPAP devices help with this issue.
As we've said, most of these machines don't cause cancer; they can even reduce the risk of cancer and other health issues.
However, some CPAP machines manufactured by Phillips have been found to elevate cancer risk. These machines contain polyester-based polyurethane (PE-PUR) sound abatement foam.
This foam is used to dampen the sound while the CPAP machine is in use. Polyurethane foam can break down and release particles and gases that can cause cancer if inhaled.
Do You Qualify for a CPAP Lawsuit?

- You used one of the recalled Philips CPAP, BiPAP, or ventilator devices for at least six months
- You have been diagnosed with cancer or a serious respiratory condition after using the device
- Your diagnosis occurred while you were using the device or within a reasonable time after use
- You file your claim within the statute of limitations for your state
- You have no significant history of the diagnosed condition prior to using the recalled device
If you've been diagnosed with breast cancer, multiple myeloma, nasal cancer, stomach cancer, brain cancer, bladder cancer, testicular cancer, liver cancer, thyroid cancer, or other cancers mentioned above, you should let your doctor know you've been using a Philips CPAP treatment.
The doctor may be able to link cancer to the CPAP breathing devices, which will help your lawsuit.
Evidence Required for a CPAP Lawsuit
To strengthen your case, you'll need to gather the following evidence:
- Proof of Device Use: Purchase records, insurance claims, or medical records showing you used a recalled Philips device.
- Medical Documentation: Records of your diagnosis, treatment history, and medical opinions linking your condition to CPAP use.
- Device Information: Serial number and model of your CPAP machine to confirm it's part of the recall.
- Usage History: Documentation showing the duration and frequency of your device use.
- Timeline: Records establishing the timeline between device use and symptom onset or diagnosis.
CPAP Lawsuit Damages
Compensation from a CPAP lawsuit can help you pay for the cancer treatment and other expenses you have.
If you're eligible for a lawsuit, the compensation you can receive should cover:
- Medical expenses — Including hospital stay, medication, and doctor appointments.
- Lost wages — If you're unable to work due to respiratory problems or cancer developed from a CPAP device.
- Loss of future earnings — If you won't be able to go back to work due to cancer or disease severity.
- Pain and suffering — If they were caused by a disease that was a result of CPAP machine use.
CPAP Recall Information
On June 14, 2021, Philips issued a voluntary recall of specific CPAP, BiPAP, and ventilator devices manufactured before April 26, 2021, due to potential health risks associated with the PE-PUR foam.
This recall affected approximately 2-3 million devices worldwide, with an estimated two-thirds sold in the United States.
Affected Devices Include:
- DreamStation CPAP and BiPAP devices
- DreamStation Go CPAP devices
- Dorma 400, 500 CPAP devices
- REMstar SE Auto CPAP machines
- Trilogy 100 and 200 ventilators
- A-Series BiPAP V30 Auto ventilators
- Several other models detailed in the FDA recall notice
Philips initially stated they would replace or repair the affected devices within a year.
However, concerns arose when testing revealed that the replacement foam also posed potential health risks. The recall and replacement process has been ongoing, with many users still waiting for replacement devices.
Each recalled Philips CPAP machine has been manufactured before April 26, 2021, and it has a serial number, so you can check if your device is part of the recall.
The FDA has classified this as a Class I recall, their most serious type, indicating "a reasonable probability that the use of these products will cause serious injuries or death."
Statute of Limitations for CPAP Lawsuits
The timeframe to file a CPAP lawsuit varies by state, typically ranging from 1-6 years from either the date of diagnosis or when you discovered (or should have discovered) that your health condition was connected to your CPAP device use.
Many states apply a "discovery rule," which means the statute of limitations begins when you knew or reasonably should have known about the link between your illness and the recalled device, not necessarily when you first used the device or when the recall occurred.
Because Philips announced the recall in June 2021, many potential plaintiffs may have only recently made the connection between their health issues and their CPAP use. It's crucial to consult with an attorney as soon as possible to ensure you don't miss the filing deadline applicable in your state.
Certain states may also have specific provisions for product liability cases or may extend deadlines for cases involving ongoing exposure to harmful substances. An experienced attorney can advise on the specific timeframes that apply to your situation.
Related Articles:
- Philips Respironics DreamStation Recall Suit
- REMStar SE Auto Recall Suit
- Philips CPAP Machine Recall Lawsuit Update
FAQs
1. Can CPAP Machine Users Seek Legal Compensation for Cancer Diagnoses?
Yes, CPAP machine users diagnosed with cancer may seek legal compensation by filing lawsuits against manufacturers, alleging failure to warn about potential risks and defects in the devices. Those who used recalled Philips devices and subsequently developed cancer or serious respiratory conditions may qualify for substantial compensation through individual lawsuits or the ongoing multidistrict litigation.
2. What Steps Are Being Taken to Ensure the Safety of CPAP Machines?
Steps include rigorous testing, FDA oversight, recalls of defective devices, and ongoing research to identify and mitigate any health risks associated with long-term CPAP machine use. The FDA has implemented enhanced testing requirements for all CPAP manufacturers, particularly focusing on the safety and stability of sound abatement materials under various conditions of use.
3. What if I Have Been Diagnosed With Cancer and I've Used a Recalled Sleep or Ventilation Device?
If you've been diagnosed with cancer and you've used a recalled sleep or ventilation device, you should consult a lawyer who can tell you if you're eligible for a CPAP lawsuit. It's important to gather all medical records and device information, as this documentation can help establish the connection between your diagnosis and the recalled device.
4. What Should I Do if My Sleep Apnea Device Has Been Recalled?
If your sleep apnea device has been recalled, you should stop using the device immediately and consult your doctor for an alternative. Also, check for any health problems that using the device may have caused, register your device with Philips to receive updates on the recall, and consider speaking with an attorney to understand your legal options.
5. How Much Compensation Might I Receive From a CPAP Lawsuit?
Compensation amounts vary based on factors including diagnosis severity, medical expenses, lost wages, age at diagnosis, and prognosis. Recent CPAP lawsuit settlements and verdicts have ranged from approximately $100,000 to over $15 million, with cancer cases typically receiving larger awards than those involving less severe respiratory conditions.
6. How Long Will My CPAP Lawsuit Take to Resolve?
CPAP lawsuits typically take 1-3 years to resolve, though this timeline can vary. Cases within the multidistrict litigation may move more quickly as bellwether trials establish precedents, while individual cases with complex medical issues or significant damages may take longer to negotiate or litigate to conclusion.
7. Can I File a Lawsuit if I Haven't Been Diagnosed With Cancer Yet?
If you haven't been diagnosed with cancer or another serious condition, you may still qualify for a medical monitoring claim if you used a recalled device. This type of claim seeks compensation for the costs of regular medical screening to detect potential health issues early. Consult with an attorney to discuss this option and monitor your health closely with your physician.
See all related medical device lawsuits our attorneys covered so far.
Get Your Free CPAP Machine Lawsuit Evaluation With Our Lawyers
Time is limited to pursue legal action for injuries related to recalled Philips CPAP machines. Most states have statutes of limitations ranging from 1-6 years from the date of diagnosis or discovery of the connection between your health condition and the defective device.
With the recall announced in June 2021, many potential claims are approaching critical deadlines. Waiting too long could permanently forfeit your right to seek compensation for medical expenses, lost income, and suffering caused by these defective devices.
The Product Liability Litigation Group at Schmidt & Clark, LLP is an experienced team of trial lawyers focused exclusively on representing plaintiffs in defective medical device lawsuits. We are currently accepting new CPAP cancer cases nationwide.
Our firm offers:
- Free, confidential case evaluations
- No upfront costs or attorney fees
- Payment only if we win your case
- Personalized attention to your specific circumstances
Don't delay seeking the compensation you deserve. Contact us today to discuss your case and legal options before time runs out.
Reference:
- https://cpapsupplies.com/blog/sleep-apnea-statistics#:~:text=50%2D70%20million%20US%20adults,standard%20for%20sleep%20apnea%20treatment.
- http://www.fda.gov/medical-devices/safety-communications/update-certain-philips-respironics-ventilators-bipap-machines-and-cpap-machines-recalled-due

 Published by
Published by 
 Skin irritation
Skin irritation Brain, breast, bladder, kidney, lung, liver, lymphatic, nasal, prostate, pancreatic, stomach, rectal, testicular, and thyroid cancers
Brain, breast, bladder, kidney, lung, liver, lymphatic, nasal, prostate, pancreatic, stomach, rectal, testicular, and thyroid cancers Toluene diamine
Toluene diamine